IRB NAVIGATION
Investigator Manual & FAQs
Does it meet the definition of Research?
HHS Definition of Research – Both must be true:- The activity is a systematic investigation: an activity that involves a prospective plan which incorporates data collection, either quantitative and/or qualitative, and data analysis to answer a question
- The activity is designed to develop or contribute to generalizable knowledge: designed to draw general conclusions (i.e., knowledge gained from a study may be applied to populations outside of the specific study population), inform policy, or generalize findings.
If the project meets the definition of Research, does it involve human subjects?
HHS Definition of Human Subject– The research involves living individuals about whom an investigator will obtain either of the following: Data through intervention or interaction with the individuals. Intervention includes both physical procedures by which data are gathered (for example, venipuncture) and manipulations of the subject or the subject’s environment that are performed for research purposes. Interaction includes communication or interpersonal contact between investigator and subject. Identifiable private information. Private information includes information about behavior that occurs in a context in which an individual can reasonably expect that no observation or recording is taking place, and information which has been provided for specific purposes by an individual and which the individual can reasonably expect will not be made public (for example, a medical record). Private information must be individually identifiable (i.e., the identity of the subject is or may readily be ascertained by the investigator or associated with the information) in order for obtaining the information to constitute research involving human subjects.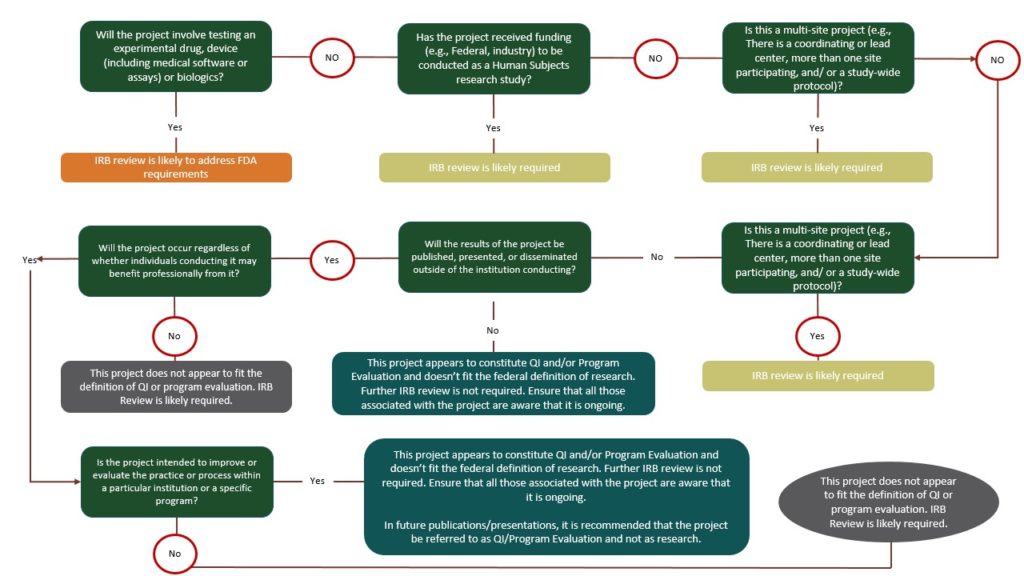
What Is It
A Clinical Trial Registry maintained by the U.S. National Institutes of Health.
Purpose
To serve as a mechanism for researchers to register clinical trials when they begin, provide timely updates, submit summary results, and make this information publicly available as part of a mechanism to provide increased stewardship, transparency, and access to clinical trials information and results.
Legal and Publication Requirements
Trial registration is required by law under Section 801 of the Food and Drug Administration Amendments Act (FDAAA 801) which requires Responsible Parties to register and submit summary results of clinical trials with ClinicalTrials.gov. The law applies to certain clinical trials of drugs (including biological products) and medical devices.
In September 2016, under an HHS/NIH initiative, changes were made to both Clinicaltrial.gov reporting requirements through changes in 42 CFR Part 11 and NIH procedures including clinical trial definition, application requirement, Good Clinical Trial (GCP) status and training requirements to enhance stewardship and improve visibility of clinical trial activities and results information. The following references summarize the related Guide notices, the new regulation, and articles published by NIH leadership in scientific journals.
- For an overview and rationale for the various clinical trial related changes, see the NIH blog, Building Better Clinical Trials through Enhanced Stewardship and Transparency
- For information on the regulation and new NIH policy on registering clinical trials and reporting summary results in ClinicalTrials.gov, see the NIH blog: Improving Visibility of NIH-supported Clinical Trial Activities and Results Information
In addition to the Final Rule and NIH policy, NIH has made available a number of resources to help explain the changes, and will be rolling out more over the upcoming months. Resources available now include:
- A summary of the Final Rule and NIH policy
- A table comparing and contrasting the Final Rule and NIH policy
- A summary table of changes to clinical trial registration and results information data elements resulting from the Final Rule
In July 1, 2005, the International Committee of Medical Journal Editors (ICMJE) decided that no trials will be considered for publication unless they are included on a clinical trials registry. Therefore even if your research is not identified as a GCP study, you may need to register your research using human subjects (even if it is behavioral study and no drug is involved) at clinicaltrials.gov in order to publish in many journals today.
Request a ClinicalTrials.Gov Account
Create an Account
Colorado State University has an organizational account. Investigators at CSU are designated as users in this university-wide account and will have their own user name and login. A member of CSU’s Quality Program serves as the Administrator and contact person for all CSU accounts.
Responsibilities
Administrator (CSU)
- Maintains PRS Organizational Account
- Creates User accounts
- Has access to all study records
- Monitors records in account for Problems
- Approves and releases records when the Organization is the
- Sponsor and Responsible Party
Users (Responsible Party): Creates and Edits Records
- Responsible for maintaining individual trial records accurately and in within required timeframes
- Only has access to their records
- Owner or on the Access List
- Approves and releases records
- Principal Investigator is typically the Responsible Party
Who Should Create an Account?
To avoid duplicate entries for a single study, studies should be registered only by the Responsible Party. Responsible parties are solely responsible for the content, quality and timeliness of registration and results reporting in accord with FDAAA. They are considered the Sponsor of the trial, the IND/IDE holder, or person or entity who initiated the trial, or funding recipient, or the funder if it is a contract.
Responsible Party
The responsible party for an applicable clinical trial (ACT) subject to FDAAA must:
- Register the ACT in ClinicalTrials.gov no later than 21 days after enrollment of the first participant;
- Update the ACT in ClinicalTrials.gov at least once every 12 month (Recruitment Status and Primary Completion Date within 30 days)
- Submit summary results (including adverse event information) for certain trials not later than 1
Provide Summary Results
All data is entered in a tabular format; no written results or conclusion
When Must Summary Results be Submitted?
- Within 12 months of (primary) completion date
- “The date that the final subject was examined or received an intervention for the purposes of final collection of data for the primar outcome, whether the clinical trial concluded according to the prespecified protocol or was terminated.”
- OR within 30 days of product approval or clearance
- Delays possible
- Seeking approval of a new use
- Extensions for “good cause”
CSU Specific Information
Social Behavioral and Educational Research IRB
IRB Name: Colorado State U IRB #1
IRB Affiliation: Colorado State University
Oversight Authorities: United States: Federal
FWA Number: 00000647 (expires 01/10/2022)
IORG (Institution/Organization) number: 0000122
IRB Number: 00000202 (expires 03/16/19)
Biomedical Research IRB
IRB Name: Colorado State U IRB #1
IRB Affiliation: Colorado State University
Oversight Authorities: United States: Federal
FWA Number: 00000647 (expires 01/10/2022)
IORG (Institution/Organization) number: 0000122
IRB Number: 00010468 (expires 03/16/19)
The CSU IRB reviews all research projects involving human subjects. The definitions of “Research” and “Human Subject” come from the US Department of Human and Health Services (HHS).
Does it meet the definition of Research?
HHS Definition of Research – Both must be true:
The activity is a systematic investigation: an activity that involves a prospective plan which incorporates data collection, either quantitative and/or qualitative, and data analysis to answer a question.
AND
The activity is designed to develop or contribute to generalizable knowledge: designed to draw general conclusions (i.e., knowledge gained from a study may be applied to populations outside of the specific study population), inform policy, or generalize findings.
If the project meets the definition of Research, does it involve human subjects?
HHS Definition of Human Subject–
The research involves living individuals about whom an investigator will obtain either of the following:
1. Information or biospecimens through intervention or interaction with the individuals. Intervention includes both physical procedures by which data are gathered (for example, venipuncture) and manipulations of the subject or the subject’s environment that are performed for research purposes. Interaction includes communication or interpersonal contact between investigator and subject; OR
2. Identifiable private information or biospecimens. Private information includes information about behavior that occurs in a context in which an individual can reasonably expect that no observation or recording is taking place, and information which has been provided for specific purposes by an individual and which the individual can reasonably expect will not be made public (for example, a medical record). Private information must be individually identifiable (i.e., the identity of the subject is or may readily be ascertained by the investigator or associated with the information) in order for obtaining the information to constitute research involving human subjects.
The project must meet the definition of both Research and Human subject to constitute Human Subjects Research.
Before conducting any Human Research, you are responsible for obtaining Institutional Review Board (IRB) approval. If the research is deemed exempt from IRB review, this determination must also be made by the institution. If you have questions about whether an activity is Human Research, contact the IRB office who will provide you with a determination. If you wish to have a written determination, please submit a Not Human Subjects Research (NHSR) Kuali protocol application.
IRBs are frequently presented with questions regarding when Quality Improvement (QI), Demonstration Projects, and other similar activities also meet the definition of human subject research and require IRB review and approval. While many QI and demonstration activities do not meet the definition of human subject research under the Common Rule or FDA, it is essential to understand that some projects may. This is the case when the QI or demonstration activity is designed to accomplish a research purpose as well as the purpose of improving the quality of care or demonstrating the success/value of a program. The following materials are included to assist the investigator and the IRB in assessing whether or not individual projects require IRB review and approval.
The intent to publish is an insufficient criterion for determining whether a QI or demonstration activity involves research. Planning to publish does not necessarily mean that the project fits the definition of research; people seek to publish descriptions of non-research activities for a variety of reasons, if they believe others may be interested in learning about those activities. Conversely, an activity may involve research even if there is no intent to publish the results.
To determine whether or not IRB review and oversight applies, the following questions should be addressed in order:
- Does the activity involve research?
- Does the research activity involve human subjects?
- Does the human subjects research qualify for an exemption? (Note: At most institutions, the authority to determine a project exempt is assigned to individuals within the Human Research Protection Program and/or IRB )
National Bioethics Commission Statement
“These activities, generally referred to as program evaluation or quality improvement, are not intended to have any application beyond the specific organization in which they are conducted. As is true in the area of public health, because populations are the subject of study and because the methods used in program evaluation or quality improvement are the same as those used in research, it is often difficult to determine whether an activity is research that falls under the oversight system.
Definitional issues regarding program evaluation or quality improvement are not limited to health care delivery. They also occur in industrial or educational settings and in social science and operations research. However, if the purpose is to assess the success of an established program, and the information gained from the evaluation will be used to improve that program, the activity should not be considered research involving human participants. Evaluation is a program monitoring tool, and the information gained will immediately benefit the program and/or the individuals involved.
However, when quality improvement involving human participants is undertaken to test a new, modified, or previously untested intervention, service, or program to determine whether it is effective and can be used elsewhere, the activity is human participant research and subject to the oversight system.”
If your project has one or more of the characteristics in the human subject research column, the project may require IRB review. If your project falls squarely into one of the other columns, then IRB review is not required.
Anonymized Data: Anonymized data may have previously been identifiable, but have since been de-identified, and a code or link no longer exists. An investigator has NO means of linking anonymized data back to a subject.
Anonymous Data: Anonymous data is data that was never linked to an individual. Coded data is not anonymous.
Coded Data: Data is coded when a link will exist between a unique code and an individual subjects’ identifiers such as name, medical record number, email address, or telephone number. Generally, the study is collected with a “STUDY ID”, and a key is maintained where the STUDY ID is associated with the subject’s identifiers. As long as a link exists, data is considered indirectly identifiable and not anonymous, anonymized, or de-identified.
De-Identified Data: A record in which identifying information is removed.
Under the HIPAA Privacy Rule, data are de-identified if:
- the data do not include any of the 18 identifiers (of the individual or his/her relatives, household members, or employers) which could be used alone or in combination with other information to identify the subject. Note that even if these identifiers are removed, the Privacy Rule states that information will be considered identifiable if the covered entity knows that the identity of the person may still be determined.
- Expert Determination Method
Private Information: Information about behavior that occurs in a context in which an individual can reasonably expect that no observation or recording is taking place, and information that has been provided for specific purposes by an individual, and that the individual can reasonably expect will not be made public (e.g., a medical record).
Examples of Identifiable Private Information
- Names
- Date of Birth
- Medical Record Numbers
- Email Addresses
- Street Addresses
- Photographs
- Audio or Video Recordings
- IP Addresses
Limited Dataset: A set of data in which most of the protected health information has been removed. The following identifiers of the individual or of the individual’s relatives, employers, or household members must be removed:
- Names;
- Addresses, other than town or city, state, and zip code;
- Telephone numbers;
- Fax numbers;
- Electronic mail addresses;
- Social security numbers;
- Medical record numbers;
- Health plan beneficiary numbers;
- Account numbers;
- Certificate/license numbers;
- Vehicle identifiers and serial numbers (including license plate numbers);
- Device identifiers and serial numbers;
- Web Universal Resource Locators (URLs);
- Internet Protocol (IP) address numbers;
- Biometric identifiers, including finger and voice prints; and
- Full-face photographic images and any comparable images.
Protected Health Information (PHI): PHI is individually identifiable health information transmitted by electronic media, maintained in electronic media, or transmitted or maintained in any other form or medium. PHI excludes individually identifiable health information in education records covered by the Family Educational Rights and Privacy Act, as amended, 20 U.S.C. 1232g, records described at 20 U.S.C. 1232g(a)(4)(B)(iv), and employment records held by a covered entity in its role as employer.
Collaborative Research
Multi-Site Research and IRB Reliance
Multi-site Research refers to human subject’s research conducted at more than one site, involving external sites that are not components of Colorado State University (CSU) (Fort Collins Campus and Spur Campus). There are different requirements for IRB review based on whether the external site or its employees are engaged in human subject’s research conducted by a CSU investigator.
To ask that CSU IRB serve as the IRB of record for a collaborative study or to request to cede oversight to another IRB of record, please contact us at [email protected].
Engagement
The IRB determines whether an external site is engaged in research using guidance from the Office of Human Research Protections guidance, “Guidance on Engagement of Institutions in Human Subjects Research.” External sites may need to consult their local IRB to determine whether their institution considers them to be engaged in the research.
IRB Reliance
When the research involves multiple sites, it’s common, and often required by the funding source for institutions to rely on one another for IRB review. CSU may enter into an IRB reliance agreement or an Institutional Authorization Agreement (IAA), which documents which institution’s IRB will serve as the IRB of record, and which institutions IRB will rely on the IRB of record.
NIH Single IRB Requirements
The Final NIH Policy on Use of a Single IRB for Multi-Site Research documents the expectation that all US sites participating in non-exempt multi-site studies funded by NIH are required to use a single Institutional Review Board (sIRB).
If your research is NIH-funded multi-site research, please reach out to the CSU IRB as soon as possible, to allow sufficient time for the reliance agreement process.
Revised Common Rule
When a CSU investigator is seeking funding from a Federal department or agency for research involving human subjects in research and their research is a non-exempt, collaborative project, they must agree to use a Single IRB. Exceptions to this rule must be approved by the funding agency program officer and the CSU IRB.
SMART IRB
CSU is a participating SMART IRB Institution. SMART IRB (Streamline, Multisite, Accelerated Resources for Trials IRB Reliance Platform) is an NIH-Funded platform designed to harmonize and streamline the IRB review process for multi-site research.
Types of Reliance Agreements
- Institutional Authorization Agreement (IAA)
- An IAA may cover all human subjects research that engages an institution or requires IRB review between two institutions or be used to document reliance on a specific study. Each institution retains the authority to determine whether to conduct its own review on a project-specific basis.
- Individual Investigator Agreements (IIA)
- IIAs are a formal agreement between CSU and an independent investigator collaborating on a CSU research study, by which CSU agrees to extend it’s (FWA) to the individual, and through which the independent investigator agrees to fulfill specified training and conduct while collaborating on the project. IIAs are subject to review and approval by CSU’s Institutional Official or designee.
Please contact the CSU IRB to inquire about reliance agreements or Individual Investigator Agreements. [email protected]
Principal Investigator Eligibility & Responsibilities
PRINCIPAL INVESTIGATOR (PI) DEFINITION
Conducting research with humans is a privilege and carries with it ethical and legal responsibilities. By Colorado State University (CSU) policy and federal regulations, the Principal Investigator (PI) is the individual responsible for writing an accurate proposal to utilize human subjects, and for designing practices and implementing the approved use(s) of those subjects. Ultimately, the PI assumes the responsibility for the ethical conduct of the project and for the welfare of the human subjects. The PI should understand and abide by all relevant federal regulations and guidelines for the use of humans in research.
CSU PERSONNEL PI ELIGIBILITY
CSU employees in the following Academic Faculty & Administrative Professional Manual appointment categories have the privilege of serving as a PI on an Institutional Review Board (IRB) protocol, if they have completed the appropriate training and experience to adequately direct the project. The IRB will consider a special request for someone out of these categories to be a PI.
- Regular Full-time
- Regular Part-time
- Multi-year Research
- Senior Teaching
- Special Appointment
- Transitional Appointment
- Administrative Professionals
- State Classified
Individuals on Temporary Appointments (e.g. Graduate Research Assistants and Postdoctoral Scholars) are not eligible to serve as PI, but can be a Co-Principal Investigator (Co-PI) with someone from one of the above categories as PI. Individuals on Visiting scholar or Adjunct appointments are not eligible to serve as PI due to the temporary nature of their affiliation with the university.
If you have a unique status that is not listed above, please contact an IRB Coordinator at [email protected].
If you have questions about whether your project needs IRB review, please reach out to us via email: [email protected]