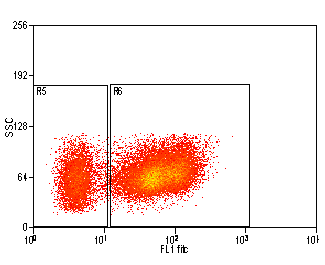
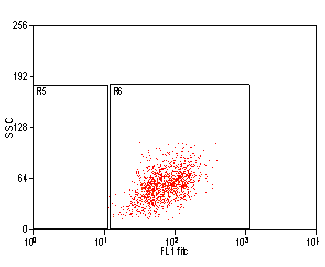
Introduction to Cell Sorting
Cell Sorting
Cell sorting involves physically separating (purifying) a specific cell population from the remaining cells or particles in the suspension for the purpose of performing further experimental procedures on these purified cells. Sterile sorted cells can be cultured to expand the population. Cells can be re-stained with other fluorescent dyes to be re-analyzed, processed for RNA studies, or other experiments to be done to characterize the purified population.
In most cell sorters, cells are passed in a stream of fluid through the flow cell; they pass through a laser beam and are analyzed in the same way as in standard flow cytometry. The sample stream is broken into droplets at a fixed break off point. If a cell of interest passes through the laser beam, it is identified, and when it reaches the droplet of the break off point, an electric charge (positive or negative) is applied to the stream. As the droplet leaves the stream it passes through deflection plates carrying a high voltage and the droplet will be attracted to one of these plates, depending on the charge it was given. Uncharged droplets pass through to the waste, and deflected droplets are collected in tubes. In this way one, two, three or four different populations of cells can be sorted from the one sample. Cells can also be sorted using a cloning attachment, which has the capability of sorting single cells (or any specified number of cells) into 96 well plates (or any other size/number of wells), or directly on to a microscope slide.
Sorting Speed
There are a number of factors that determine the length of the cell sort, including cell type, cell size, cell concentration, sticky or clumpy cells, sort mode (purity or enrichment), and frequency (%) of the target cells.
Sorting speed is generally around 10,000 – 12,000 cells per second using the 100 micron flow cell tip for larger or sticky cells, and around 25,000 cells/second for smaller, well-suspended cells using the 70 micron flow cell tip.
Expected yields are usually 40% of the ideal yield on average. Highly purified cells may be less due to the number of aborted droplets. Higher yields are possible with less purity or enrichment.
Any BSL 2 material, including tissue from healthy human donors, is sorted with the aerosol evacuation unit running, and this adds some time to the sort. The sorting chamber must be evacuated before removing the sorted cells.
Post-sort Purity
The purity of each sorted population will be tested after each sort by re-running the sorted population. . The purity of each sort can be compromised by several factors
- Unwanted cells (or platelets/debris) may be aggregating with cells of interest. These unwanted particles can separate after the sort, resulting in the contamination of a number of unwanted particles
- Loss of viability after the sort may result in cells exhibiting different light scatter properties from those of the original cells.
- The capping of cell surface markers after the sort will often result in sorted cells that are somewhat less fluorescent than the original selected population.
- Dead cells can non-specifically bind antibody and thus can appear to the flow cytometer as positive events. The flow cytometer can discriminate most dead cells, but cells that have recently died or are not healthy can lead to false positive results.
- The cells of interest are not completely resolved from the unwanted cells (overlapping populations)